Cellulosic ethanol – The basics: Conversion pathway - Thermochemical
Section: ADVANCED BIOFUELS
Series: Cellulosic
ethanol
- The
basics: Conversion pathway – Thermochemical
Posts: CELLULOSIC ETHANOL
1. Introduction
Currently, the thermochemical conversion
pathway for converting biomass resources into ethanol occupies a subsidiary
position. This approach has received modest levels of support in the past. Nevertheless,
it is worthwhile reviewing the concept in the framework of this series about
cellulosic ethanol.
The pathway involves two major steps:
(1) Gasification of the lignocellulosic biomass to generate syngas.
(2) Transformation of the syngas in ethanol.
This second step can be carried out by chemical catalysts or fermentation (hybrid route). In contrast to chemical catalytic
conversion, fermentation conversion can produce pure ethanol as opposed to a
mixture of alcohols.
Advantages
- All of the organic matter within the feedstock
is broken down, which results in the release of a higher proportion of carbon
for ethanol production.
- Gasification is suitable for all biomass sources,
thermochemical conversion can use a wider range of feedstocks than biochemical
conversion. It is not adversely affected by lignin in the biomass. In fact, it is
mostly appropriate for forest feedstocks and wastes rich in lignin.
- It requires fewer processing chemicals.
Disadvantages
- There is little scope for valorization of
co-products since all of the biomass is converted.
- Biomass feedstock moisture content heavily
influences alcohol yields and emissions.
- The process is complex and temperatures
during gasification are relatively high.
- Raw syngas contains catalyst and fermentation
contaminants that must be removed before alcohol production.
The general process areas include: feedstock
preparation, gasification, gas cleaning and conditioning, ethanol production
and purification. Many
possible configurations exist for each conversion approach: there are several gasification
technologies as well as ethanol synthesis options.
2. Feedstock preparation
The size in which the feedstocks have been
harvested needs to be reduced to the level where it is easy to handle and the
process becomes more efficient. For instance, agricultural wastes need to be
grinded and forest wastes have to be taken through a chipping process in order
to reach a uniform size. Also, the biomass is dried from the as-received moisture
to that required for proper feeding into the gasifier.
3. Gasification
Gasification is the exothermic partial
oxidation of biomass with process conditions optimized for high yields of
gaseous products (synthesis
gas, syngas or producer gas). It involves the devolatilization and conversion
of biomass in an atmosphere of steam and/or oxygen. The crude synthesis gas is
primarily composed of CO, H2, CO2, CH4, tars
and water.
There are two general classes of gasifiers:
(1) Partial oxidation (POX) gasifiers
(directly-heated gasifiers). They use the exothermic reaction between
oxygen and organics to provide the heat necessary to devolatilize biomass and to
convert residual carbon-rich chars. In POX gasifiers, the heat to drive the
process is generated internally within the gasifier. A disadvantage of this kind
of gasifiers is that oxygen production is expensive and typically requires
large plant sizes to improve economics.
(2) Steam gasifiers (indirectly-heated
gasifiers). They accomplish biomass heating and gasification through heat
transfer from a hot solid or through a heat transfer surface. Either byproduct
char and/or a portion of the product gas can be combusted with air (external to
the gasifier itself) to provide the energy required for gasification. Steam gasifiers
have the advantage of not requiring oxygen; but since most operate at low pressure,
they require product gas compression for downstream purification and synthesis
unit operations.
4. Gas cleanup and conditioning
This stage consists of multiple units:
- Reforming of tars and other hydrocarbons
to CO and H2.
One of the challenges of gasification is the
management of higher molecular weight volatiles that condense into tars, which are
both a fouling challenge and a potential source of persistent environmental
pollutants such as PAH. They can be reformed into useful syngas using a fluidizable
catalyst.
- Syngas quench.
The hot syngas is cooled through heat exchange
with the steam cycle and additional cooling via water scrubbing. The scrubber also
removes impurities such as particulates and ammonia along with any residual
tars.
- Acid gas (CO2 and H2S)
removal.
The cooled syngas enters an amine unit to
remove the CO2 and H2S. Later, the H2S is reduced
to elemental sulphur.
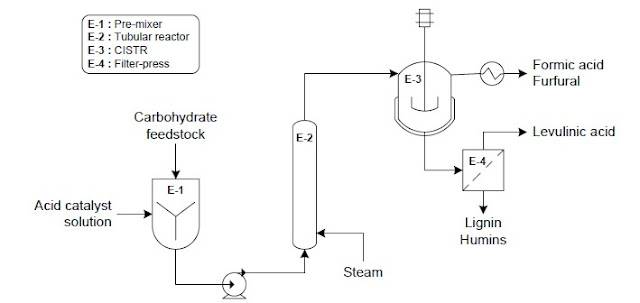
5. Alcohol production and separation
5.1 Chemical catalysis of syngas
The cleaned and conditioned syngas is converted
to alcohols in a fixed bed reactor. The syngas is further compressed to the
required synthesis pressure and sent through a fixed-bed molybdenum-sulfide-based
catalyst to synthesize a variety of mixed alcohols.
After synthesis, the alcohols are cooled and
condensed away from the unconverted syngas. The mixture is cooled through heat exchange
with the steam cycle and other process streams. The condensed alcohols undergo
distillation and purification to recover pure ethanol. The depressurized
alcohol stream is dehydrated using vapor-phase molecular sieves. Methanol is recovered
and recycled to the synthesis reactor in order to boost ethanol yields.
Figure 1. Thermochemical conversion – Chemical
catalysis of syngas (extracted from Reference [3])
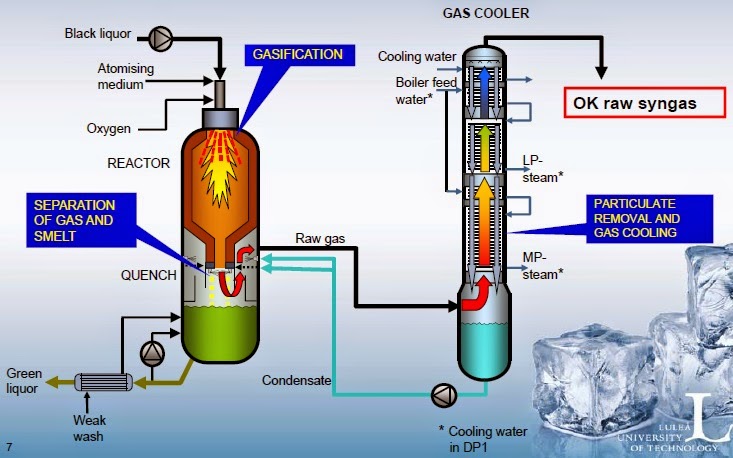
5.2 Fermentation of syngas (hybrid route)
The clean and conditioned syngas is fed to
fermentation where it is converted to ethanol. The resulting fermentation broth
is quite dilute, typically containing 2% or less of ethanol. The ethanol can be
recovered from the broth using conventional recovery schemes. A simple
gas-sparged tank reactor, operating in batch or continuous mode, can be used for
the fermentation.
The micro-organisms used for ethanol production
from syngas mixtures are anaerobes that use a heterofermentative version of the
acetyl-CoA pathway for acetogenesis. The acetyl-CoA intermediate is then
converted into either acetic acid or ethanol as a primary metabolic product. In
contrast to many other syngas-based processes, syngas fermentation performance
is not tied to a specific ratio of H2 to CO. While the organisms
generally prefer CO to H2, both CO and H2/CO2
mixtures can be simultaneously converted.
Figure 2. Thermochemical conversion – Fermentation
of syngas (extracted from Reference [6])
6. Cases studies: biorefineries at
commercial scale
Case study: Enerkem (gasification
+ chemical catalysis)
The Enerkem biorefinery in Edmonton is the
first commercial-scale plant in the world to produce cellulosic ethanol from
non-recyclable, non-compostable mixed municipal solid waste (MSW).
The plant was officially opened in June 2014
and began to produce and sell biomethanol since 2016. A new
methanol-to-ethanol conversion unit was installed in 2017 and the production
of ethanol started in September of that year.
Entrada: “Enerkem begins production of
cellulosic ethanol from MSW at its Edmonton biorefinery”, 18/9/2017.
|
|
INEOS New Planet BioEnergy plant was constructed for demonstrating at
full commercial scale the economic conversion of a variety of different
lignocellulosic waste biomass feedstocks to bioethanol and renewable
electricity utilizing the INEOS Bio technology. In addition to having the
capacity to produce 8 Mgal (30 Ml) per year of ethanol, the plant also could
generate up to 6 MW of electricity.
The construction was completed in June 2012 and the first production
of cellulosic ethanol at commercial scale took place one year later. In
December 2014, the plant was shut down for the installation of a HCN
scrubber. The presence of low levels of hydrogen cyanide, toxic to the
organisms involved in the fermentation, was a major problem for the process.
In 2016, the NREL reported (2015 Survey of
Non-Starch Ethanol and Renewable Hydrocarbon Biofuel Producers) that the plant was idled in 2015 while working on mechanical
improvements and was expected to resume operations sometime this year.
Finally, in September 2016, Ineos Bio announced its intention to sell its
ethanol business, including the New Planet BioEnergy plant.
|
____________________________________________________________________________________________________________________________________________
References
[1] P.L. Spath, D.C. Dayton: “Preliminary
Screening – Technical and Economic Assessment of Synthesis Gas to Fuels and
Chemicals with Emphasis on the Potential for Biomass-Derived Syngas”. Technical
Report NREL/TP-510-34929, December 2003.
[2] S. Phillips, A. Aden, J. Jechura, D. Dayton:
“Thermochemical Ethanol via Indirect Gasification and Mixed Alcohol
Synthesis of Lignocellulosic Biomass”. Technical Report
NREL/TP-510-41168, April 2007.
[3] T.D. Foust, A. Aden, A. Dutta, S. Phillips:
“An economic and environmental comparison of a biochemical and a thermochemical
lignocellulosic ethanol conversion processes”. Cellulose, 16:547–565, June
2009.
[4] A. Dutta et al.: “Process Design and
Economics for Conversion of Lignocellulosic Biomass to Ethanol. Thermochemical
Pathway by Indirect Gasification and Mixed Alcohol Synthesis”. Technical
Report NREL/TP-5100-51400, May 2011.
[5] Daystar et al.:“The NREL Biochemical and
Thermochemical Ethanol Conversion Processes: Financial and Environmental
Analysis Comparison”. BioResources 10(3), 5096-5116, July 2015
[6] M. Devarapalli, H.K. Atiyeh: “A review of
conversion processes for bioethanol production with a focus on syngas fermentation”.
Biofuel Research Journal 7 (2015) 268-280.