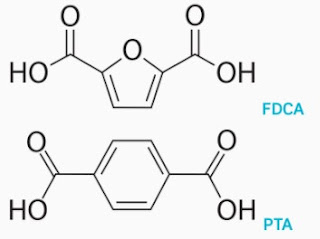
FDCA (2,5-furandicarboxylic acid) biorefineries
Publication date:
15/06/2017
Last update: 24/01/2018
Introduction 1,2,3,4,5
2,5-Furandicarboxylic
acid (FDCA), also known as dehydromucic acid and pyromucic acid, is an organic
compound that was first detected in human urine. In fact, a healthy human produces
3-5 mg/day. It is a very stable compound. Some of its physical properties, such
as insolubility in most of common solvents and a very high melting point (it
melts at 342 °C), seem to indicate intermolecular hydrogen bonding. FDCA has
two carboxylic acid groups, which makes it a suitable monomer for polycondensation
reactions with diols or diamines.
It is one of the
top 12 value-added biobased chemicals listed by the US DoE in 2004. The list
was updated in 2010 and FDCA was included again, but this time in a group together
with furfural and 5-hydroxymethylfurfural (5-HMF). Those three molecules are
the main representatives of the furanics (furan derivatives) that has been
referred to as “Sleeping Giants” because of their enormous market potential. In
recent years, FDCA has received significant attention due to its wide
application in many fields, particularly as a substitute of
petrochemical-derived terephthalic acid in the synthesis of useful polymers.
Process technologies 1,2,3,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22
FDCA was first
prepared from mucic acid by Fittig and Heinzelmann in 1876 by reacting with
fuming hydrobromic acid under pressure. Currently, the most common route for
producing FDCA is through 5-HMF oxidation, which in turn is traditionally
produced by dehydrating hexoses, especially fructose. The conversion can be
performed via an acid catalyzed dehydration reaction in supercritical acetone,
water with phase modifiers or high boiling solvents. 5-HMF is not stable and
degrades upon storage. It may undergo re-hydration in aqueous phase, thereby
originating byproducts such as levulinic and formic acid or even condensate
into polymers called humins. So, the use of stable intermediates (for instance,
alkoxy-derivatives) or the direct conversion of fructose into FDCA in one pot
are preferred.
Numerous processes
have been studied and are described in detail in literature. For instance,
biological transformations and electrochemical routes have been reported
recently. In the table beneath, you will find a non-extensive overview of the
most important processes developed or under research by companies and research
institutes. Most of the patents and web pages consulted are very recent. It is
a clear illustration of the great interest generated by the compound. The table
will be updated in the future.
Main Route
|
Company / Resarch institution
|
Previous step
|
Starting material
|
Specific characteristics of the process
|
Catalytic oxidation.
|
-
|
5-HMF.
|
Medium: water.
Catalytic system: homogeneous, water-soluble metal
salt catalyst.
|
|
Hydrothermal Processing (HTP) of sugars (fructose 90+ %) to
produce 5-HMF. Currently, they are using 1G
sugar but the process is ready to use 2G sugar anytime it becomes commercially
available at competitive prices.
|
5-HMF.
|
Oxidation.
5-HMF is oxidized
to obtain FDCA. There are a number of different technologies for the oxidation
available (biological, enzymatic, chemical).
Finally, a
purification step is necessary before go into the polymerization process to produce PEF or other polymers. |
||
Catalytic dehydration of the carbohydrate feedstock
in an alcohol to make Alkoxymethyl-Furfural (RMF).
|
Alkoxymethyl-Furfural (RMF).
|
Medium: acetic acid.
|
||
-
|
5-HMF, 5-HMF esters, 5-HMF ethers and 5-alkyl
furfurals.
|
Oxidant agent: O2.
Key intermediate: 5-formyl furan-2-carboxyic acid
(FFCA).
Medium: acetic acid.
Catalyst system: Co/Mn/Br.
|
||
-
|
5-HMF.
|
Oxidant agent: O2.
Medium: water.
Catalyst system: catalyst containing a metal of the
platinum group.
|
||
Aqueous-phase saccharides dehydration.
|
5-HMF.
|
Catalytic system: heterogeneous supported metal
catalyst.
|
||
Disproportionation reaction of furoic acid salts.
|
-
|
Furfural compounds.
|
Catalysed by metal salts to produce a mixture of
2,4-FDCA and 2,5-FDCA.
The furoic acid salts are obtained by oxidizing
furfural compounds in the presence of catalysts and alkaline solution.
|
|
Biocatalytic conversion.
|
-
|
5-HMF.
|
-
|
|
Acid hydrolysis of biomass.
|
-
|
-
|
-
|
|
Dehydroxylation.
|
Oxidation of galacturonic acid (a constituent of
pectin) with a fungal biocatalyst.
|
Aldaric acids.
|
Dehydroxylation of the aldaric acid into furan
carboxylic acid (FCA) and FDCA or muconic acid depending on the reaction
conditions (see post).
|
|
Electrochemical oxidation.
|
-
|
5-HMF.
|
The method employs solar cells.
|
Applications 1,3,5,6,7,8,12,23
FDCA can be used for a range of applications, including green chemicals and biopolymers. Despite its chemical stability, it undergoes reactions typical for carboxylic acids, giving carboxylic dihalides, esters and amides. The materials market represents a multi-billion-euro business and includes plastics, plasticizers, thermosets and coatings.
Below, main
applications are shortly described:
- Polyesters,
polyamides and polyurethanes
The most important
group of FDCA conversions is undoubtedly the polymerisation. The FDCA monomer
offers great opportunities to create a wide range of polymers: polyesters (bottles,
containers and films), polyamides (for new nylons) and polyurethanes.
PEF is featured
further down.
- Plasticizers
FDCA esters have
recently been evaluated as replacements for phthalate plasticizers for PVC.
- Fire foams
FDCA, as most of
polycarboxylic acids, is an ingredient of fire foams. Such foams help to
extinguish fires in a short time caused by polar and non-polar solvents.
- Precursor of
levulinic and succinic acids
All the
applications of these platform molecules.
- Pharmacology
FDCA has been
largely applied in pharmacology. It was demonstrated that its diethyl ester had
a strong anaesthetic action similar to cocaine. Screening studies on some FDCA
derivatives showed important anti-bacterial properties. A diluted solution of
FDCA in tetrahydrofuran is utilised for preparing artificial veins for
transplantation.
PEF
Polyethylene furanoate
(PEF) deserves a specific space in this applications chapter. The most
important polyester is PET (polyethylene terephthalate) which is produced using
purified terephthalic acid (PTA) and ethylene glycol (EG). The market for
virgin PET is currently around 50 million tons per year. The main raw material
for PTA is para-xylene (PX) which is generated by oil refining. EG, the other
building block for PET production, is obtained on the basis of ethylene, made
by oil cracking. EG is also produced from bioethanol and extensive efforts are
being made to commercialize PX from renewable sources.
However, in
addition to the production of building blocks that are chemically identical to
existing petrochemical building blocks, it is also possible to make entirely
new monomers based on biobased raw materials. FDCA can replace PTA to obtain
PEF in large applications such as bottles and carpets. When also using
renewable EG, a 100% renewable PEF can be produced. As FDCA has a different
molecular structure than PTA, the resulting polymer will also have other
properties. In spite of this fact, they are sufficiently similar to allow FDCA
to be used in combination with EG in existing PET polymerization plants, making
FDCA an infrastructure drop-in. In a similar manner, PEF can also be used in
downstream conversion plants. Furthermore, PEF is recyclable which offers
converters and brandowners the opportunity of a closed loop product lifecycle.
In fact, the European PET Bottle Platform (EPBP) has recently given interim approval
for the recyclability of PEF to be produced by Synvina in the European bottle
recycling market.
With regard to
thermal properties, PEF has a better performance than PET as it has a higher
thermal stability (higher glass transition temperature) combined with a lower
processing temperature (lower melting point). PEF is also seen as a superior
material for bottles due to its increased gas barrier properties. In addition,
PEF opens the door to new applications where PET properties do not suffice,
like in smaller serving sizes and light-weighting and also for replacing other
packaging materials like glass and aluminum cans.
Biorefineries at commercial scale and demo plants 14,24,25
At the time of writing, only two companies have
announced the construction of commercial scale FDCA biorefineries. Albeit, due
to the growing interest on this building block, it is foreseeable that more
companies follow their steps in the next few years. Below, a summary of the
characteristics and status of the facilities at commercial scale and demo plants
that are operating or under planning.
Commercial-scale facilities – Under planning
|
|||||
Owner
|
Location
|
Feedstocks
|
Technology
|
Capacity
|
Status
|
Synvina (Avantium and BASF Joint Venture).
|
BASF’s Verbund site in Antwerp (Belgium).
|
-
|
Avantium XYX process.
|
50,000 tons/year.
|
BASF and Avantium announced in October 2016 the
formation of a new JV for the production and marketing of FDCA produced from
renewable resources (see post). To
realize this ambition, they are planning to construct a reference plant (TRL
8).
An industry consortium called “PEFerence”,
coordinated by Synvina, has been granted with 25 M€ for establishing a
complete value chain for the use of FDCA for PEF (see post,
09/06/2017). The construction of the plant is included in the scope of the
project.
The reference plant is planned to be operational in the 2023-2024 timeframe (see post, 16/01/2018).
|
AVA Biochem
|
-
|
-
|
AVA Biochem process.
|
Phase 1 is planned to be 30,000 tons/year and is set
to increase to 120,000 tons/year at full capacity.
|
AVA Biochem is
planning to start a first commercial production plant for 5-HMF and maybe FDCA
by 2020.
5-HMF technology
is ready for commercialization (TRL 8-9). FDCA technology is currently at 4-6
depending on the technology to be used.
First PEF products
will be jointly-produced and tested with globally active partners from the
value chain.
A first financing
round for the plant’s engineering work has already been completed. |
Demo facilities - Operating
|
|||||
Owner
|
Location
|
Feedstocks
|
Technology
|
Capacity
|
Status
|
Avantium
|
Chemelot Campus in Geleen (Netherlands)
|
Avantium XYX process.
|
40 tons/year.
|
In December 2011, Avantium officially opened its
pilot plant. Designed in conjunction with partners to optimize resources and
expertise, the pilot started up successfully and is currently running 24/7.
| |
Note: AVA Biochem owns a pilot/demo scale of 50 kg/hour name plate capacity for 5-HMF technology. FDCA oxidation is not yet a pilot scale. However, they can use the existing AMOCO process which has been used for decades for the PTA production.
Demo facilities – Under construction
|
|||||
Owner
|
Location
|
Feedstocks
|
Technology
|
Capacity
|
Status
|
Origin Materials
|
Western Sarnia-Lambton Research Park (Canada)
|
-
|
Eastman.
|
-
|
In September 2017, Eastman Chemical Company and
Origin Materials entered into a non-exclusive agreement for Eastman to
license its proprietary FDCA and FDCA derivatives production technology from
renewable resources to Origin Materials. Also, Origin Materials purchased an
oxidation pilot plant from Eastman. The whole project will include the
relocation, commissioning and process validation of a pilot plant (see post,
09/06/2017).
|
__________________________________________________________________________________________________________________________________
REFERENCES
1 J. Lewkowski: “Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives”. ARKIVOC 2001 (i) 17-54.
REFERENCES
1 J. Lewkowski: “Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives”. ARKIVOC 2001 (i) 17-54.
2 S. P. Teong, G. Yi, Y. Zhang: “Hydroxymethylfurfural production from bioresources: past, present and
future”. Green Chemistry, 2014, 16, 2015–2026.
3 T. Werpy, G.R. Petersen: “Top Value Added Chemicals from Biomass. Volume 1: Results of Screening for Potential Candidates from Sugar and Systhesis Gas”. US DoE, August 2004.
4 J.J. Bozell, G.R. Petersen: “Technology development for the production of biobased product from biorefinery carbohydrates – the US Department of Energy’s Top 10 revisited”. Green Chemistry, 2010, 12, 539–554.
5 E. de Jong, M.A. Dam, L. Sipos, G.-J.M. Gruter: “Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters”. ACS Symposium Series, Vol. 1105. Biobased Monomers, Polymers, and Materials. Chapter 1, pp 1–13. August 16, 2012.
6 “Bio-Based Chemicals: Value Added Products from Biorefineries”. IEA Bioenergy, Task 42 Biorefinery.
7 P. Harmsen, M. Hackmann: “Green Building Blocks for Biobased Plastics”. Wageningen UR Food & Biobased Research, March 2013.
8 C.H.R.M. Wilsens: “Exploring the application of 2,5-furandicarboxylic acid as a monomer in high performance polymers :synthesis, characterization, and properties”. Eindhoven: Technische Universiteit Eindhoven DOI: 10.6100/IR783770, 2015.
9 Z. Zhang and K. Deng: “Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives”. ACS Catal., 2015, 5 (11), pp 6529–6544.
10 M. Gattinger et al.: “Cyclization and Dehydration of Aldaric Acids to 2,5-Furandicarboxylic Acid”. 2016 AIChE Annual Meeting.
11 G.S. Hossain1 et al.: “Metabolic engineering of Raoultella ornithinolytica BF60 for the production of 2, 5-furandicarboxylic acid from 5-hydroxymethylfurfural”. AEM Accepted Manuscript Posted Online 21 October 2016, Appl. Environ. Microbiol. doi:10.1128/AEM.02312-16.
12. A. Sanborn: “Process for making 2,5-furandicarboxylic acid”. Patent: US 9562028 B2 (ADM), 07/02/2017.
13 AVA Biochem web page (accessed on 27/05/2017).
14 Avantium web page (accessed on 27/05/2017).
15. J. van HAveren et al.: “Process For The Production Of The Mixture 2,4 Furandicarboxylic Acid (FDCA) And 2,5 Furandicarboxylic Acid Via Disproportionation Reaction, Mixture Of 2,4-FDCA And 2,5-FDCA As A Result Of Disproportination Reaction, 2,4-FDCA Obtained By The Disproportionation Reaction Process And Use Of 2,4-FDCA”. Patent: US20150119588 A1 (Braskem), 30/05/2015.
16 “FDCA bioplastics”. Corbion Purac FDCA brochure.
17 J. Mesfin et al.: “Oxidation process to produce a crude and/or purified carboxylic acid product”. Patent: US 20150011783 A1 (Eastman), 08/01/2015.
18 G. Borsotti et al.: “Process for the synthesis of 2,5-furandicarboxylic acid”. Patent: US 20130137882 A1 (Novamont), 30/05/2013.
19 “Mercurius Biorefining and University of California, Davis to Develop Technology for Low-Cost FDCA Production”. Mercurious Biorefinig press release, 31/08/2016.
20 B.G. Siqueira et al.: “2.5-furandicarboxylic acid integrated production process”. Patent: US 9199957 B2 (Petrobras), 01/12/2015.
21 “A new method for producing plant-based drinking bottles from FDCA”. VTT press release, 03/05/2017.
22 “Green plastics from citrus fruit peels and sugar”. The making of tomorrow, VTT.
23 “Researchers develop new approach that combines biomass conversion, solar energy conversion”. WARF news, 10/03/2015.
24 “Synvina receives interim approval from European PET Bottle Platform: PEF to be integrated in circular economy”. Synvina Press Release, 22/05/2017.
25 K. Laird: “AVA-CO2 announces successful development of new interface for different FDCA oxidation routes”. Plastics Today, 25/05/2016.
3 T. Werpy, G.R. Petersen: “Top Value Added Chemicals from Biomass. Volume 1: Results of Screening for Potential Candidates from Sugar and Systhesis Gas”. US DoE, August 2004.
4 J.J. Bozell, G.R. Petersen: “Technology development for the production of biobased product from biorefinery carbohydrates – the US Department of Energy’s Top 10 revisited”. Green Chemistry, 2010, 12, 539–554.
5 E. de Jong, M.A. Dam, L. Sipos, G.-J.M. Gruter: “Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters”. ACS Symposium Series, Vol. 1105. Biobased Monomers, Polymers, and Materials. Chapter 1, pp 1–13. August 16, 2012.
6 “Bio-Based Chemicals: Value Added Products from Biorefineries”. IEA Bioenergy, Task 42 Biorefinery.
7 P. Harmsen, M. Hackmann: “Green Building Blocks for Biobased Plastics”. Wageningen UR Food & Biobased Research, March 2013.
8 C.H.R.M. Wilsens: “Exploring the application of 2,5-furandicarboxylic acid as a monomer in high performance polymers :synthesis, characterization, and properties”. Eindhoven: Technische Universiteit Eindhoven DOI: 10.6100/IR783770, 2015.
9 Z. Zhang and K. Deng: “Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives”. ACS Catal., 2015, 5 (11), pp 6529–6544.
10 M. Gattinger et al.: “Cyclization and Dehydration of Aldaric Acids to 2,5-Furandicarboxylic Acid”. 2016 AIChE Annual Meeting.
11 G.S. Hossain1 et al.: “Metabolic engineering of Raoultella ornithinolytica BF60 for the production of 2, 5-furandicarboxylic acid from 5-hydroxymethylfurfural”. AEM Accepted Manuscript Posted Online 21 October 2016, Appl. Environ. Microbiol. doi:10.1128/AEM.02312-16.
12. A. Sanborn: “Process for making 2,5-furandicarboxylic acid”. Patent: US 9562028 B2 (ADM), 07/02/2017.
13 AVA Biochem web page (accessed on 27/05/2017).
14 Avantium web page (accessed on 27/05/2017).
15. J. van HAveren et al.: “Process For The Production Of The Mixture 2,4 Furandicarboxylic Acid (FDCA) And 2,5 Furandicarboxylic Acid Via Disproportionation Reaction, Mixture Of 2,4-FDCA And 2,5-FDCA As A Result Of Disproportination Reaction, 2,4-FDCA Obtained By The Disproportionation Reaction Process And Use Of 2,4-FDCA”. Patent: US20150119588 A1 (Braskem), 30/05/2015.
16 “FDCA bioplastics”. Corbion Purac FDCA brochure.
17 J. Mesfin et al.: “Oxidation process to produce a crude and/or purified carboxylic acid product”. Patent: US 20150011783 A1 (Eastman), 08/01/2015.
18 G. Borsotti et al.: “Process for the synthesis of 2,5-furandicarboxylic acid”. Patent: US 20130137882 A1 (Novamont), 30/05/2013.
19 “Mercurius Biorefining and University of California, Davis to Develop Technology for Low-Cost FDCA Production”. Mercurious Biorefinig press release, 31/08/2016.
20 B.G. Siqueira et al.: “2.5-furandicarboxylic acid integrated production process”. Patent: US 9199957 B2 (Petrobras), 01/12/2015.
21 “A new method for producing plant-based drinking bottles from FDCA”. VTT press release, 03/05/2017.
22 “Green plastics from citrus fruit peels and sugar”. The making of tomorrow, VTT.
23 “Researchers develop new approach that combines biomass conversion, solar energy conversion”. WARF news, 10/03/2015.
24 “Synvina receives interim approval from European PET Bottle Platform: PEF to be integrated in circular economy”. Synvina Press Release, 22/05/2017.
25 K. Laird: “AVA-CO2 announces successful development of new interface for different FDCA oxidation routes”. Plastics Today, 25/05/2016.